The photoelectric effect has been well known since the publication of Albert Einstein’s 1905 paper explaining that quantized particles of light can stimulate the emission of electrons from materials. The nature of this quantum mechanical effect is closely related to the question how much time it might take for an electron to leave a material such as a helium atom. The exciting news at JILA is that the Ultrafast AMO Theory Group has come up with a clever way that may help to answer this question by observing a photoelectron on its way out of, but still inside, an atom.
The theorists show how a combination of attosecond (10-18 s) and femtosecond (10-15 s) laser pulses could be used in the laboratory to follow the electrons inside a helium atom on an ultrafast time scale. Such an experiment would open the door to observations of the behavior of electrons inside different atoms and molecules during the photoelectric effect. This seminal work appeared in an article published online December 24, 2014, in Physical Review Letters.
The researchers responsible for proposing the use of ultrafast laser pulses to really see something happening inside an atom include recently minted JILA Ph.D.s Jing Su and Hongcheng Ni as well as Fellows Agnieszka Jaron-Becker and Andreas Becker.
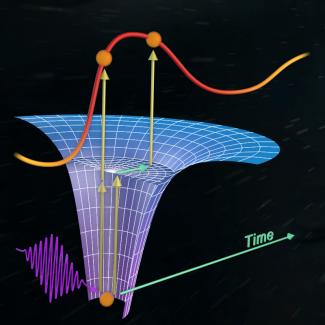
Their secret was to use a streaking camera, in which the variation of the photoelectron’s energy with time is measured by the combination of two different laser pulses. First, one or two photons from the attosecond laser pulse kick the electron out of its ground state inside the atom. Then, the photoelectron interacts and oscillates in the electric field of the second longer femtosecond laser pulse. The femtosecond laser field changes the energy of the electron depending on the time for the photoelectric effect to happen. This allows the researchers to probe the electron’s behavior inside the atom (or molecule) during the photoelectric effect.
For example, if an attosecond photon kicks the electron first into resonance with one of the higher energy states in the atom, then the electron hangs out a while in the excited state before a second photon finally pushes the electron all the way out of the atom. The femtosecond laser pulse in the streaking camera measures the time the electron takes to move through and leave the atom.
In contrast, if the photon of the attosecond laser doesn’t kick a helium electron into resonance, the electron is immediately pushed out of the atom. The femtosecond pulse instantaneously captures this behavior. In this way, the streaking camera may make it possible for researchers to observe and follow the kicking of an electron into a resonance state in real time. They may also be able to answer the question of how much it prolongs the photoelectric process inside the atom.
Jaron-Becker, Becker, and their colleagues have opened the door to watching the inner workings of an atom during the photoelectric absorption and emission process. Now they have to wait and see if experimentalists can actually accomplish this feat in the laboratory. In the meantime, the Ultrafast AMO Theory Group is beginning work on using a streaking camera to peer inside other atoms and molecules. Stay tuned.—Julie Phillips